Cross Sections, Part I
In our story so far we have atoms, proven to exist with a known size, established by none other than Einstein himself. We have sub-atomic particles, electrons, orbiting at a considerable distance from the protons and neutrons that comprise a densely packed atomic nucleus. We also know that if we hurl neutrons at Uranium the Uranium may split or fission. We also have a good many European, Jewish, scientists who have reason not only to flee in terror but to fear that nowhere is truly safe for them if those who they flee develop a weapon based on fission first.
There was still very real questions; why did uranium fission sometimes and could a nuclear chain reaction be sustained? Clearly, this did not happen in the natural world of the 20’th century Earth, but was there some way to make it happen synthetically? Enter the brilliant Nobel laureate for physics in 1922, Niels Henrik David Bohr, or rather re-enter, Bohr was the man who had helped Lise Meitner escape Nazi Germany.
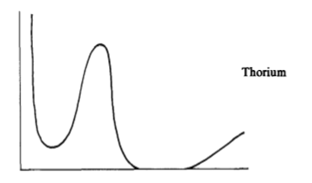
Bohr, already well known for his work in quantum mechanics and the emissions of energy from excited atoms, had conceived a ‘liquid drop model’ of the atomic nucleus in 1936, which he used to explain neutron absorption. There was a problem, it was recognized that the element Thorium had very similar neutron absorption and elastic cross sectional properties as Uranium yet for some reason only Uranium fissioned (and only sometimes) when bombarded by ‘slowed’ (thermalized as physicists call it today) neutrons. On Sunday February 5, 1939 while Bohr and Léon Rosenfeld were staying at the Nassau Club, the Princeton faculty center, George Placzek joined them. Placzek challenged Bohr on his liquid drop model. How could this model be valid given the difference in behavior between thorium and uranium to slowed neutrons?
Luckily Rosenfeld provided an account of the event to Richard Rhodes:
Bohr abruptly saw why and was struck dumb. Not to lose what he had only barely grasped, oblivious to courtesy, he pushed back his chair and strode from the room and from the club. Rosenfeld hurried to follow, “Taking a hasty leave of Placzek, I joined Bohr, who was walking silently lost in deep meditation, which I was careful not to disturb.
When Bohr reached his office, (borrowed from Einstein, the most respected scientist by that time wanted a much more modest space and took a secretarial annex). Bohr proceeded to draw three diagrams on a chalkboard; the diagrams showed the neutron cross section on the Y-axis and the neutron energy on the X-axis. The first two diagrams were identical for both thorium 232 and uranium 238, In both cases thorium and uranium absorb thermal neutrons at energies of about 25 electron volts (eV) but it turns out they also fission if the neutrons are really energetic (with energies above a million eV or 1 MeV). The first two diagrams were of two different cross sections, a neutron absorption and a fission cross section. Furthermore the more energetic the neutrons, above 1 MeV, the more likely a fission will happen, in an apparent linear relation, that is a 1 MeV neutron is very unlikely to fission a U-238 or Th-232, but a, say 14.1 MeV neutron is very likely to induce fission.
On February 7 Bohr submitted a paper to the ‘Physical Review’, nearly 2000 words long, “Resonance in uranium and thorium disintegrations and the phenomenon of nuclear fission”, was at first regarded almost as curiosity, most scientists as Enrico Fermi (1938 Nobel laureate) would later recall, viewed isotopes as “almost magically inseparable”. There was also controversy Bohr had produced a theory, it would have to be validated experimentally, many including Fermi rejected Bohr’s explanation. Finally in every observation of nuclear fission, neutrons had come from an external source and struck uranium. It was believed that neutrons were freed, based on the masses of the fission products, but would these neutrons go on to fission more uranium atoms and thus begin a chain reaction?
Of course today we know the answer to these questions, that’s how people in Ontario get electricity to read this article or charge their electric car, but between the late winter and spring of 1939 the greatest minds in physics were consumed by thoughts of chain reactions and the possibility that what had once been dismissed as “moonshine” might actually be something very different.



Comments
Post a Comment